Moderna Vaccine Wikipedia | Pfizer Biontech Covid 19 Vaccine Wikipedia
Moderna Inc tidigare Moderna Theurapeutics är ett amerikanskt läkemedels- och bioteknikföretag baserat i Cambridge Massachusetts. Durch die Impfung gelangt dieser Bauplan über kleinste Fettpartikel.
The United States Food and Drug Administration gave Moderna permission to test the vaccine again in more people.
Moderna vaccine wikipedia. Long-term storage temperature. The Moderna COVID-19 Vaccine is still being studied in clinical trials. Impfstoffe wie Spikevax Vaccine Moderna von Moderna enthalten Genabschnitte des SARS-CoV-2-Virus in Form von messenger-RNA messenger-Ribonukleinsäure kurz mRNA.
Call the vaccination provider or your healthcare provider if you have any side effects that bother you or do not go away. The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations. Företagets enda kommersiella produkt är COVID-19 Vaccine Moderna.
Moderna grundades 2010 och utvecklar läkemedel baserade på budbärar. These include potential new mRNA medicines for treating infectious diseases cancer rare diseases and cardiovascular disease. Report vaccine side effects to FDACDC Vaccine Adverse Event Reporting.
That means it is being tested on humans. The Moderna mRNA-1273 vaccine was authorized by the Food and Drug Administration for emergency use during the ongoing COVID-19 pandemic in December 20201 Out of 15185 participants in the phase III Coronavirus Efficacy COVE trials who received at least one dose of the Moderna vaccine 228 15 reported hypersensitivity adverse events including injection site. If you experience a severe allergic reaction call 911 or go to the nearest hospital.
The Moderna vaccine entered stage-three clinical trials on June 27 2020. Diese wird auch als Boten-RNA bezeichnet. Januar 2020 meddelte Moderna om udviklingen af en vaccine til hæmning af COVID-19 coronavirus.
Moderna is working to create mRNA medicines for a wide range of diseases and conditions. Modernas teknologi er en messenger RNA-forbindelse compound kaldet mRNA-1273 som hæmmer SARS-CoV-2 der koder for en form af virussens spike-proteinVaccinekandidaten er af den RNA-baserede vaccinetype og udvikles i et samarbejde mellem Moderna og US National Institute of. The PfizerBioNTech COVID-19 vaccine can be kept between 25 and 15 C 13 and 5 F for up to two weeks before use and between 2 and 8 C 36 and 46 F for up to five days before use.
Sowohl der von BioNTech und Pfizer entwickelte Impfstoff Tozinameran als auch das von Moderna entwickelte Vakzin mRNA-1273 geben den Körperzellen eine mRNA -Vorlage zur Herstellung des Spike - Proteins von SARS-CoV-2 siehe RNA-Impfstoff. The second dose of the PfizerBioNTech and Moderna vaccines can be administered up to six weeks after the first dose to alleviate a shortage of supplies. Sie enthält quasi einen Bauplan für ein Merkmal des Coronavirus.
Modernas chief medical officer said the vaccine could be ready in January 2021. What should I do about side effects. Moderna utvecklar bland annat vaccin.
Janssen Covid 19 Vaccine Wikipedia
Covid Vaccine Pfizer Says 94 Effective In Over 65s Bbc News

Moderna Covid 19 Vaccine Wikipedia

Covid Vaccines Johnson Johnson S Shot Authorized By F D A The New York Times

Moderna Covid 19 Vaccine Wikipedia

File Covid Vaccine 41 50753217957 Cropped Jpg Wikimedia Commons

Covid 19 Vaccination In India Wikipedia

Pfizer Biontech Covid 19 Vaccine Wikipedia
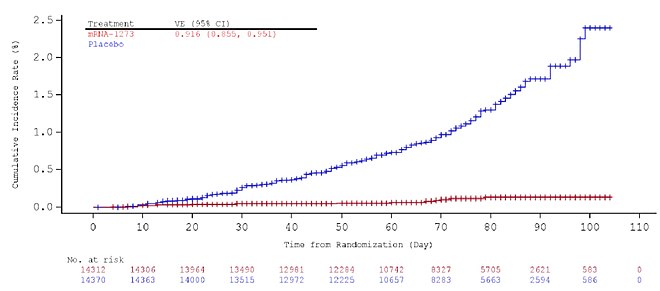
Moderna Covid 19 Vaccine Wikipedia

Covid 19 Vaccination In Bangladesh Wikipedia
Covid 19 Chinese Vaccine Successful In Mid Stage Trials Bbc News

How Does Russia S Covid 19 Vaccine Compare With Pfizer Moderna Bloomberg

Coronavirus Update Aug 6 2021 Moderna Claims Its Vaccine Is 93 Percent Effective After Six Months Prague Czech Republic
How The U S Government Bolstered Moderna S Covid 19 Vaccine Candidate Drug Discovery And Development




Comments
Post a Comment